Treating wide-neck and complex intracranial aneurysms remains one of the biggest challenges in neurovascular intervention. While traditional coiling has long been the standard, its effectiveness drops significantly when aneurysms have wide necks, shallow domes, or unusual shapes. To address these limitations, intrasaccular devices have been developed to provide a stand-alone method for disrupting intra-aneurysmal blood flow, encouraging safe thrombosis, and supporting long-term occlusion — all while preserving the parent artery.
This article explains how intrasaccular devices work inside the aneurysm sac, the key design elements that influence their flow-disrupting performance, and how surface coatings can fine-tune this delicate balance between thrombosis promotion and vessel safety.
How Intrasaccular Devices Work
Unlike coils, which are packed individually into an aneurysm, intrasaccular devices are self-expanding implants made of fine braided mesh or structured frameworks. Once deployed inside the sac, these devices create a physical scaffold that disrupts the incoming blood flow vortex. By slowing down and redirecting intra-aneurysmal circulation, the device initiates clot formation inside the sac — the first step toward stable aneurysm occlusion.
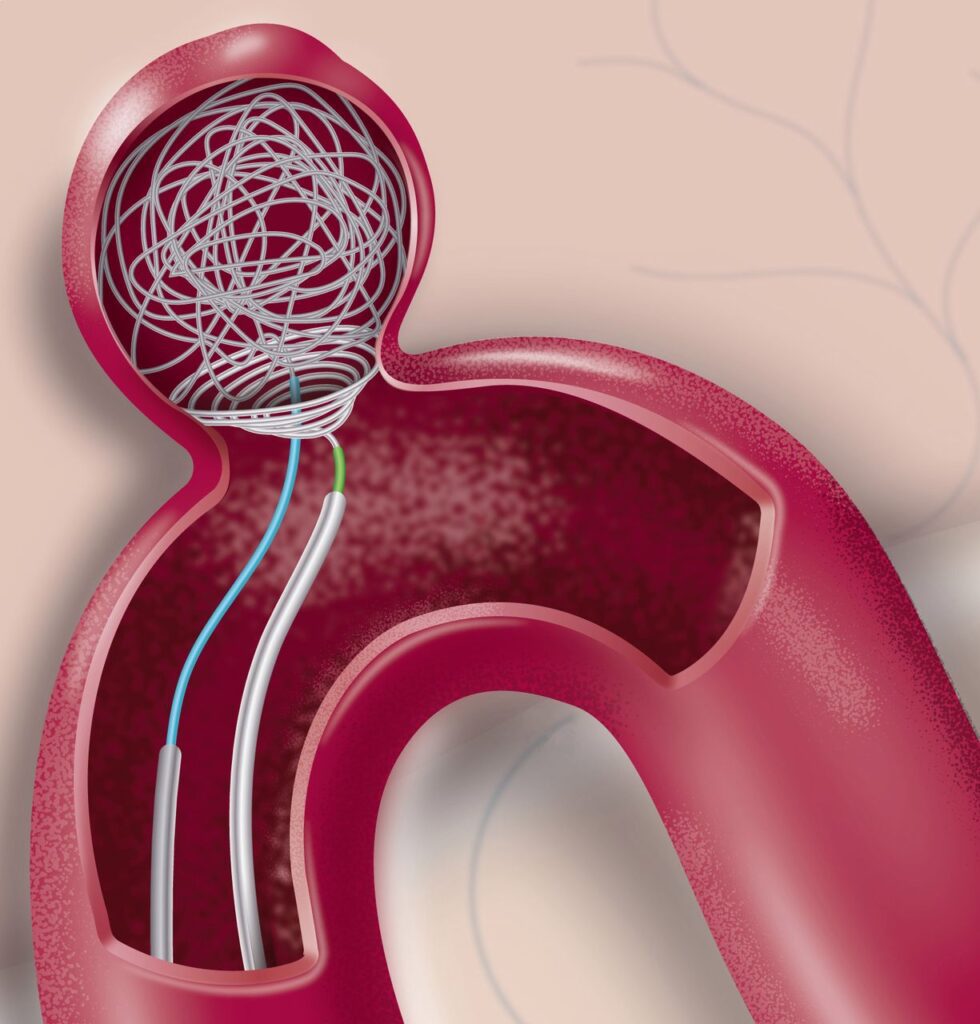
A well-designed intrasaccular device achieves three main objectives:
- Disrupt Inflow: Break the high-velocity jet of blood entering the sac.
- Stabilize Clot Formation: Create a low-flow zone where thrombus can organize.
- Preserve Parent Vessel Flow: Prevent significant flow disruption in the main artery.
The balance between these effects is critical. If intra-sac clotting is too weak, the aneurysm may remain partially patent, increasing rupture risk. If clotting spills into the parent artery, thromboembolic complications can occur.
Key Design Features
Modern intrasaccular devices use advanced design elements to achieve controlled flow disruption:
- Braided Mesh Structure: Fine wires woven into a dense mesh reduce intra-aneurysmal flow while allowing the device to expand naturally inside irregular sac shapes.
- Pore Density and Porosity: The size and distribution of the mesh pores determine how much blood can enter the sac and where flow slows enough to support clot formation.
- Shape Conformability: Self-expanding structures ensure that the device contacts the sac wall fully, minimizing gaps where flow could persist.
- Radiopacity: Markers integrated into the braid help position the device accurately to ensure complete neck coverage.
Devices like the Woven EndoBridge (WEB) and newer plug-style implants have shown how these features come together to make stand-alone aneurysm treatment more effective and predictable.
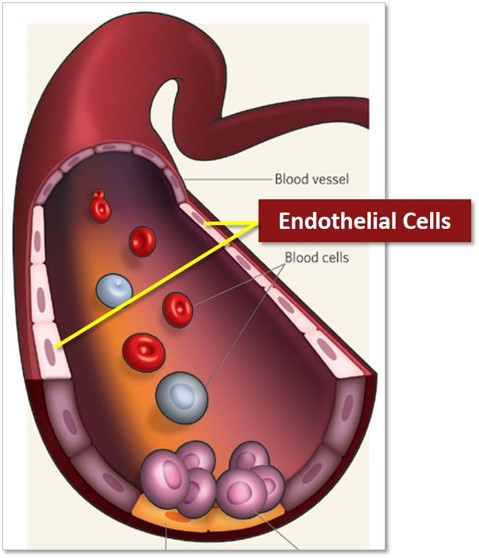
The Role of Surface Coatings in Flow Disruption
While design features shape the physical flow environment, the surface properties of the device determine how blood interacts with it at the micro-level. The goal is to ensure that the device promotes localized thrombosis inside the sac while discouraging clot formation where it could harm the parent vessel.
Advanced surface coatings like Camouflage™ by Smart Reactors are engineered to help fine-tune this balance
- Hemocompatibility: By reducing unnecessary platelet activation on the exposed surface, coatings help keep clotting localized to the sac interior.
- Endothelial Bridging: Once thrombosis occurs, the next step is healing the aneurysm neck. A coating that supports endothelial cell adhesion encourages a stable tissue layer to grow over the neck opening, permanently sealing the defect.
- Reduced Inflammation: A biocompatible coating can also limit chronic inflammation, which helps maintain device stability and reduces the risk of late-stage complications.
Bringing Mechanism and Healing Together
The mechanical design and the biological surface must work together for the device to deliver durable results. While the device shape and mesh structure manage blood flow inside the sac, a bioactive coating helps manage the biological response — improving occlusion rates and minimizing risks for the patient.
To see how Smart Reactors’ Camouflage™ coating complements the flow-disrupting power of intrasaccular devices, read our related feature:
Optimizing Intrasaccular Devices: The Role of Advanced Surface Coatings.
Next-generation aneurysm care depends on more than device shape alone. It requires a deep understanding of hemodynamics, thrombosis control, and the biological interface between blood and implant. Combining smart design with advanced coatings helps ensure that intrasaccular devices perform exactly where they need to — inside the sac, protecting patients where it matters most.
Share this post: on LinkedIn