Hemocompatibility
Hemocompatibility with Camouflage™ Coatings
In the world of medical implants, hemocompatibility is not just a requirement—it’s a necessity. Devices that come into contact with blood must function seamlessly, without triggering complications like clotting, inflammation, or immune responses. Camouflage™ coating is designed to dramatically improve the hemocompatibility of your medical devices, ensuring they meet the highest standards of safety and performance. Camouflage™ has been tested for many blood markers per guidance in ISO-10993-4.
Camouflage™ has consistently delivered exceptional results across a spectrum of key blood markers, setting a new benchmark for what’s possible in device hemocompatibility and redefining expectations for implant performance.
Devices that lack hemocompatibility can trigger serious complications, such as:
- Blood Clots (Thrombosis): The formation of blood clots can block vital blood flow, leading to potentially life-threatening conditions.
- Inflammation: Poor hemocompatibility can result in inflammation, prolonging recovery and leading to adverse reactions.
- Immune Response: Devices that are not hemocompatible may cause the immune system to attack the foreign object, compromising its function and lifespan.
Case Study: Hemocompatibility of Camouflage™ Coated Neurovascular Implants
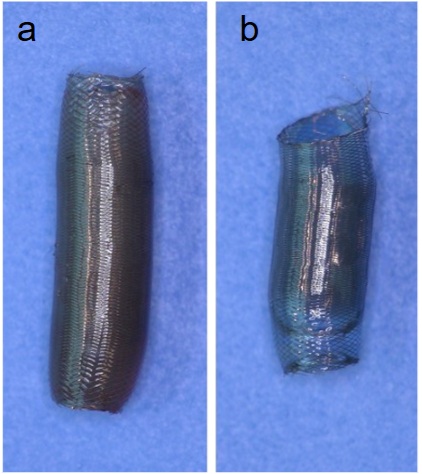
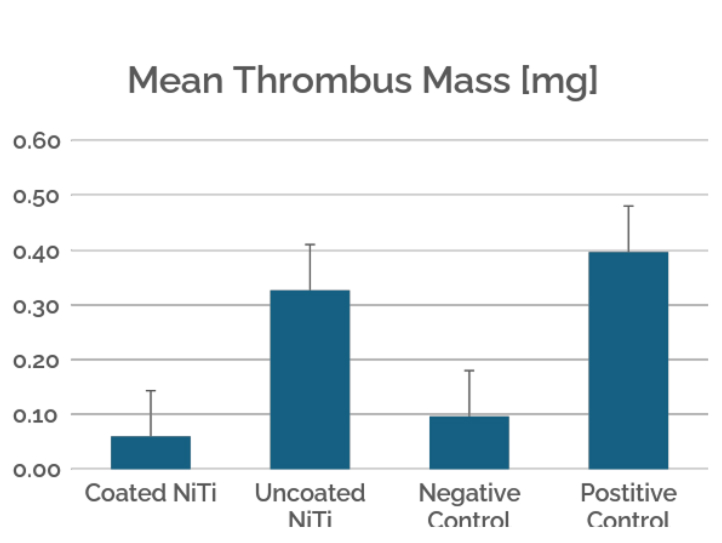
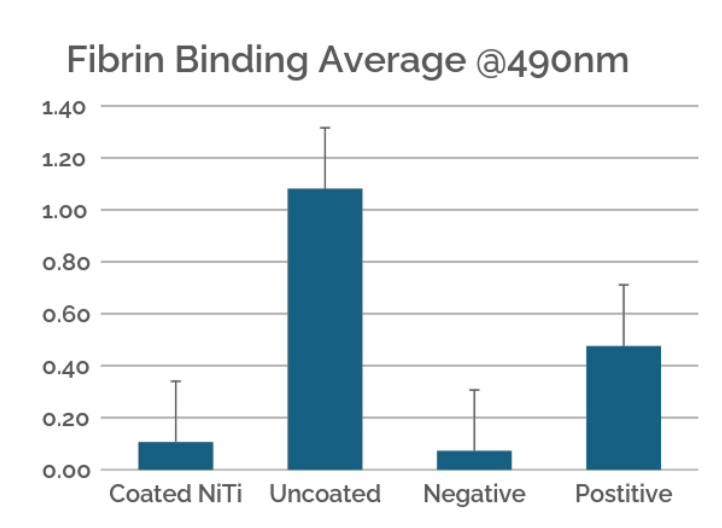
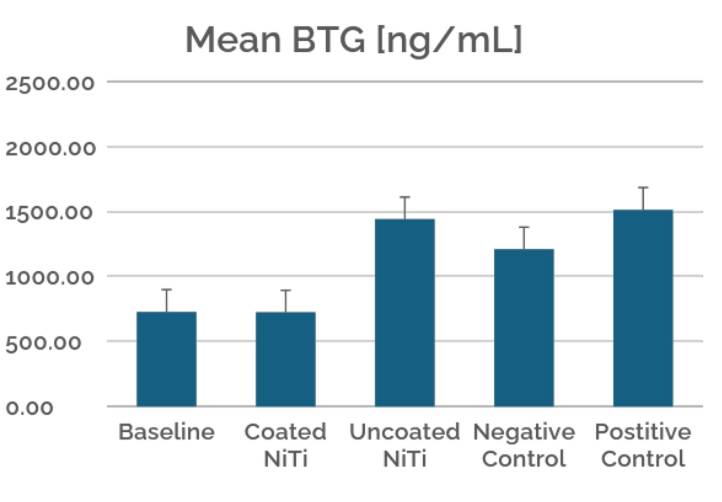
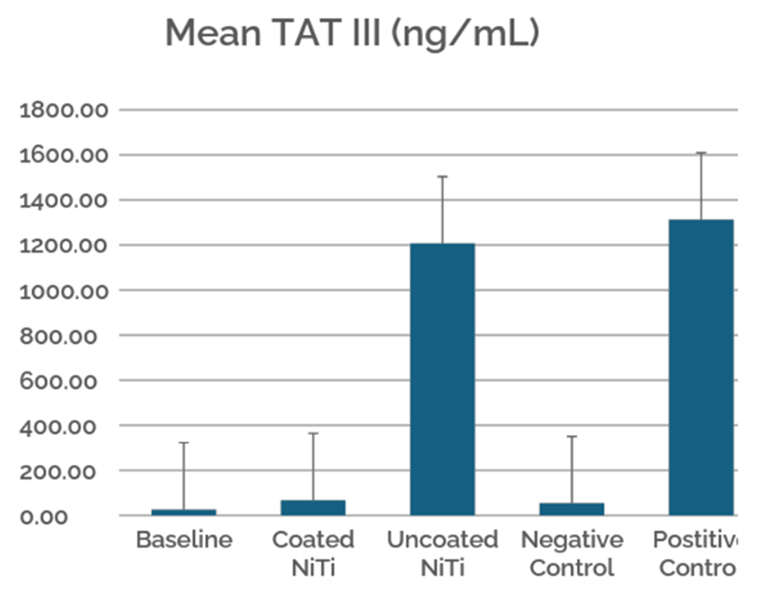
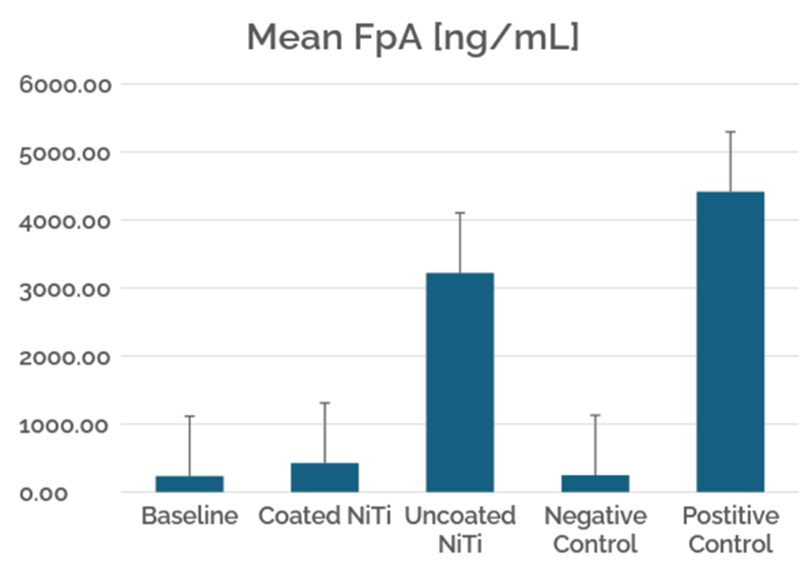
This case study evaluates the hemocompatibility of neurovascular flow diverters coated with Smart Reactors’ Camouflage™ coating technology. As flow diverters remain in direct contact with circulating blood, hemocompatibility is critical to their performance. Test samples were exposed to whole human blood under controlled in vitro flow conditions. Following exposure, surfaces were assessed for blood component deposition and biomarkers of blood activation to understand coating–blood interactions relevant to thrombotic and inflammatory responses.
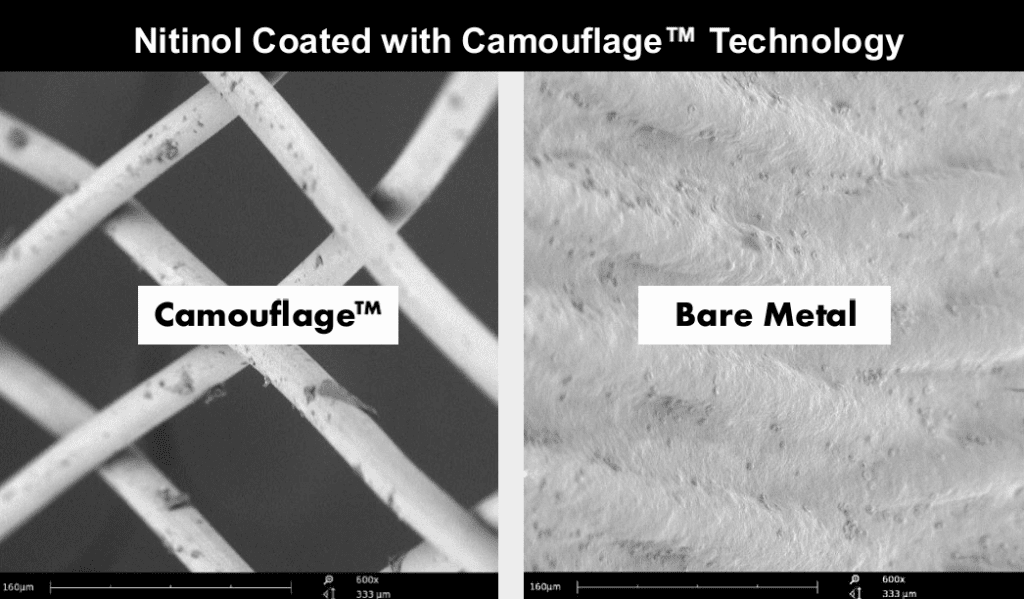
Figure 1. SEM (600x) of NiTi flow-diverter coated with Camouflage “V” uncoated- 60mins chandler Loop
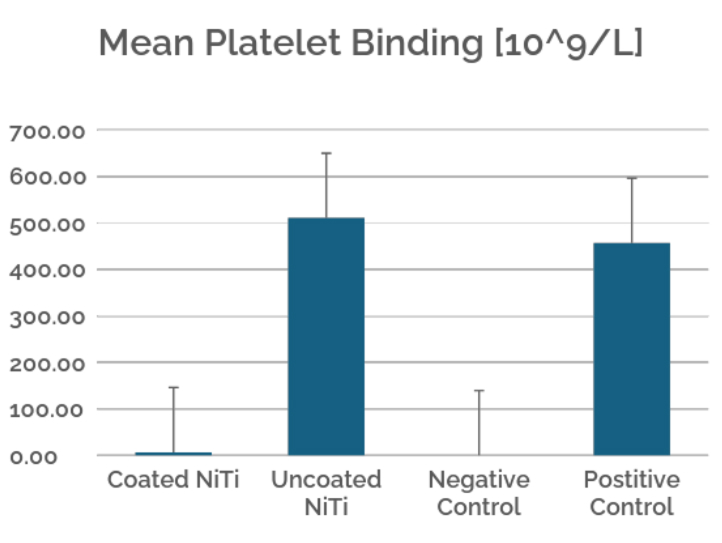
Figure 3. Platelet binding was determined by the amount of acid phosphatase (platelet release enzyme) present on the sample surface. The coated nitinol group showed the lowest platelet binding levels, demonstrating a significantly more hemocompatible surface when treated with Camouflage
Figure 5. Fibrin binding was determined by the amount of labelled anti-fibrin antibodies that adhered to the surface. Nitinol coated with Camouflage indicated a lower level of fibrin binding than uncoated nitinol.
Risks Associated with Non-Hemocompatible Implantable Devices
Devices that lack hemocompatibility can trigger serious complications, such as:
Blood Clots (Thrombosis): The formation of blood clots can block vital blood flow, leading to potentially life-threatening conditions.
Inflammation: Poor hemocompatibility can result in inflammation, prolonged recovery and increase the risk of adverse reactions.
Immune Response: Devices that lack hemocompatibility may cause the immune system to attack the foreign object, compromising its function and lifespan.
Camouflage™ Advantage in Hemocompatibility
Camouflage™ coating is specifically designed to tackle hemocompatibility issues, offering a range of benefits that can help to ensure your medical devices are as safe and effective as possible.
Reduced Thrombosis Risk: By promoting a smooth and blood-compatible surface, Camouflage™ minimizes platelet adhesion, significantly reducing the risk of thrombosis. This is essential for blood-contacting devices such as stents, heart valves, and vascular grafts.
Minimized Immune Reactions: Camouflage™ coating is engineered to be biocompatible, helping devices integrate smoothly into the body without triggering excessive immune responses.
Improved Long-Term Stability: This coating reduces inflammation, ensuring that the implant remains functional and stable in the body for longer periods, reducing the need for revision surgeries.
Faster Recovery Time: The hemocompatible surface created by Camouflage™ accelerates the body’s natural healing process, leading to faster recovery times and fewer complications for patients.
How Camouflage™ Enhances Hemocompatibility
Camouflage™ functions by selectively binding non-inflammatory proteins from the patient’s blood to the surface of the device. This helps to create a protective layer that minimizes the risk of adverse bodily reactions, ensuring better integration and reducing immune responses.
Camouflage™ coating technology utilizes an optimized surface that interacts with blood to prevent complications such as:
Platelet Repulsion: The surface of Camouflage™ is engineered to repel platelets, which are responsible for initiating blood clot formation. This helps to ensure that blood flows smoothly across the device without clotting.
Controlled Protein Adsorption: Camouflage™ allows non-inflammatory proteins such as albumin to be retained on the device surface which effectively conceals the underlying material from the risk of clot formation.
Why Hemocompatibility Matters for Your Devices
When it comes to blood-contacting devices, hemocompatibility is directly tied to patient safety and product success. Devices that are not hemocompatible can cause serious complications, leading to increased healthcare costs, revision surgeries, and, in severe cases, loss of life. By ensuring your devices are treated with Camouflage™, you can offer:
Enhanced Patient Safety: Minimized risk of thrombosis and inflammation.
Extended Device Lifespan: Reduced complications lead to fewer revisions surgeries and extended functionality.
Regulatory Compliance: Camouflage™ coating complies with stringent requirements for medical device safety and performance in blood-contacting applications.
Get Started with Camouflage™ Today
Are you ready to take your medical devices to the next level with Camouflage™? Contact us today to learn more about how our hemocompatible coatings can help you achieve better performance, safety, and patient outcomes. Get in touch with us to request a demo or discuss your specific application needs.
Why Choose Camouflage™?
Camouflage™ offers medical device manufacturers a cutting-edge coating solution that directly addresses hemocompatibility challenges. Choosing Camouflage™ means choosing:
Safety: Reduced risk of life-threatening complications such as thrombosis and immune reactions.
Performance: Long-term device functionality with fewer complications.
Patient Trust: Devices that integrate seamlessly with the body leading to better patient outcomes and satisfaction.