Endothelialization
Camouflage™ promotes Endothelialization
The integration of surface coatings, such as Camouflage™, aims to mitigate medical device complications by enhancing both hemocompatibility and endothelialization, allowing for smoother recovery and better device integration. There are real risks in untreated surfaces, however, Camouflage™ coatings provide a robust solution.
Implantable devices typically function in two phases which involve thrombus formation and endothelialization:
1. Thrombus Formation Phase
Platelets in the bloodstream become activated, which is essential for thrombus (clot) formation. Over days to weeks, a stable thrombus develops within the serving as a scaffold for further healing. While necessary, this phase carries the risk of clot propagation and device-related thrombosis if poorly managed.
2. Endothelialization Phase
Over months to years, the thrombus transforms into collagen. During this phase, endothelial cells, which line the blood vessels integrate it into the vascular system. This endothelial layer is critical to the long-term success of the devices, preventing complications such as clot formation.
3. Challenges of Bare Metal Surface
Bare metal surfaces attract proteins that trigger clotting, inflammation, and thrombosis. Without a protective coating, they interact with clotting factors, raising the risk of stroke.
Preclinical Evaluation of Camouflage™ Coated Stents in a Rabbit Carotid Model
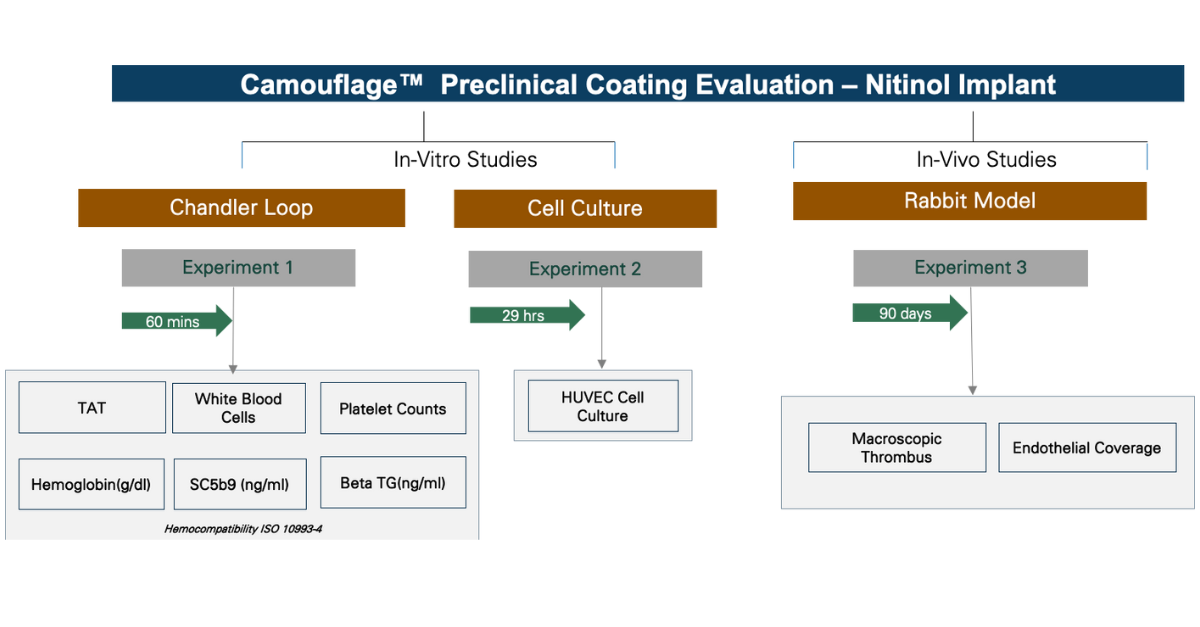
This preclinical study was designed to assess the safety and endothelialization performance of Camouflage™ coated stents compared to uncoated controls in a rabbit carotid artery model. One coated stent was implanted in the left common carotid artery (LCCA), and two stents, one coated and one uncoated, were implanted in the right common carotid artery (RCCA).
Animals were sacrificed at three time points: 24 hours (n=1), 14 days (n=2), and 90 days (n=1). Of the two animals evaluated at Day 14, one received dual antiplatelet therapy (DAPT; aspirin and clopidogrel), while all other animals underwent testing without antiplatelet treatment.
Following euthanasia, stented vessel segments were explanted and fixed in formalin for 24 hours. The segments were then longitudinally opened to expose the inner lumen for visual assessment.
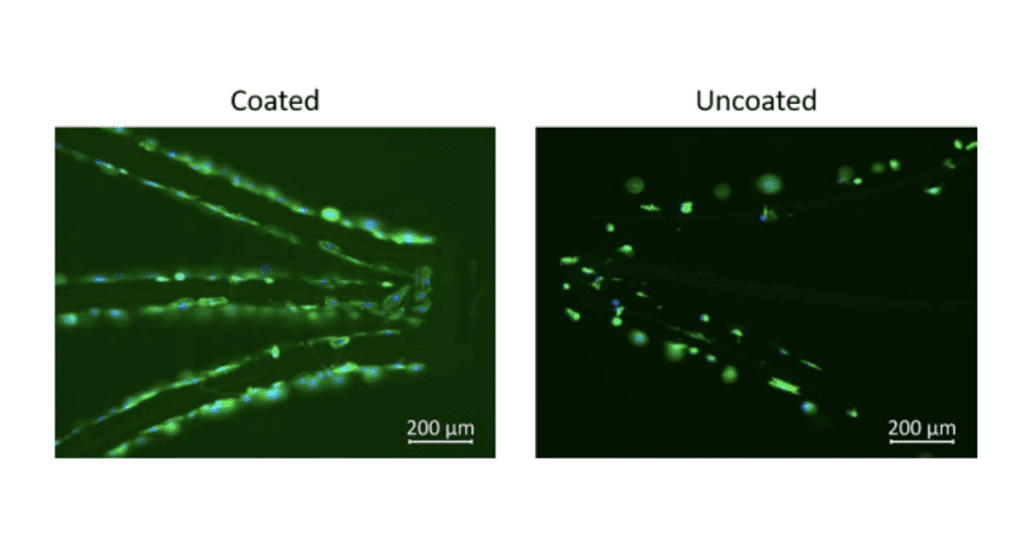
Endotheliazation-Nitinol Stents
Fig 1 & 2 are evidence of Camouflage™ coating on nitinol stents and below demonstrates the increased attachment of endothelial cells to Nitinol surfaces coated with camouflage™ compared to controls.
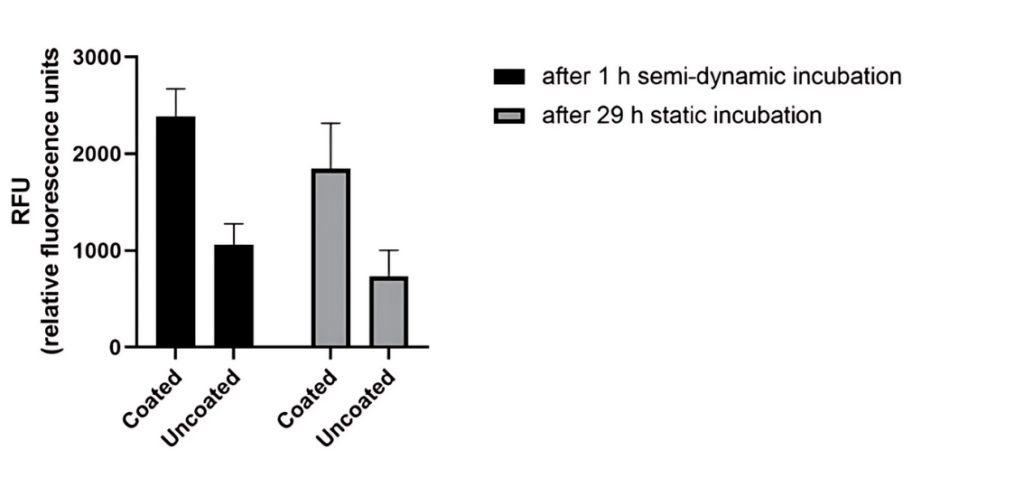
Endothelialization- Cell viability
Viability of cells on coated and uncoated NiTi stents after 1hr semi-dynamic incubation and after 29hr static incubation.
Preclinical Rabbit Results
- All animals remained healthy throughout the study period with no observed complications.
- No technical issues were encountered with any of the devices.
- Camouflage™ coated stents demonstrated more rapid Endothelialization compared to uncoated stents upon gross inspection of the inner lumen.
These findings support the favorable biocompatibility and healing response of Camouflage™ coating in vivo.
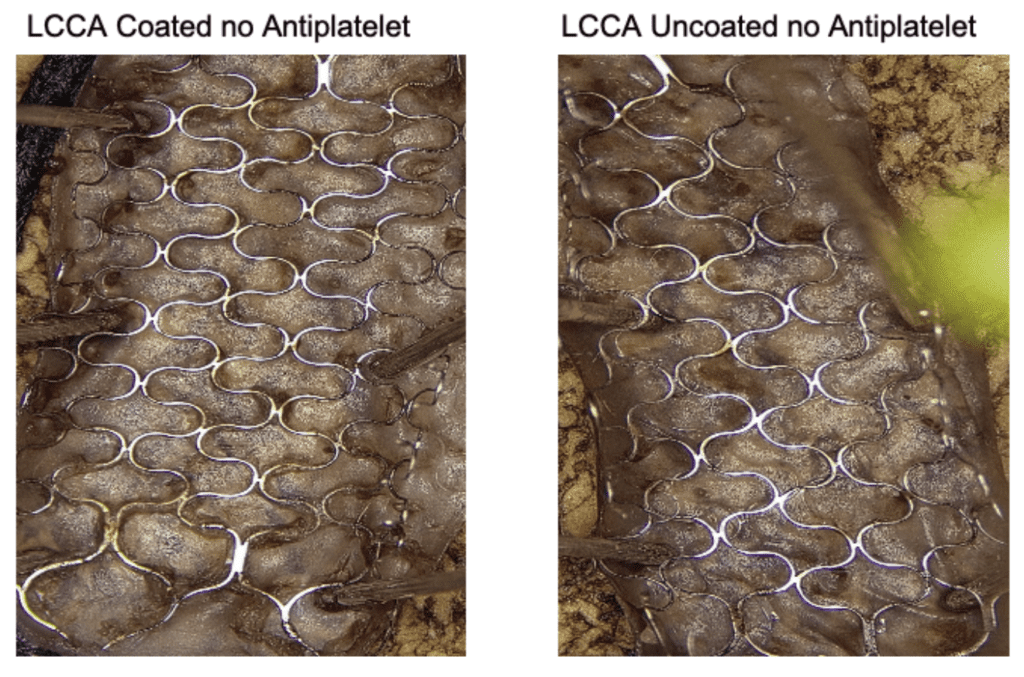
24 hours Post Implantation
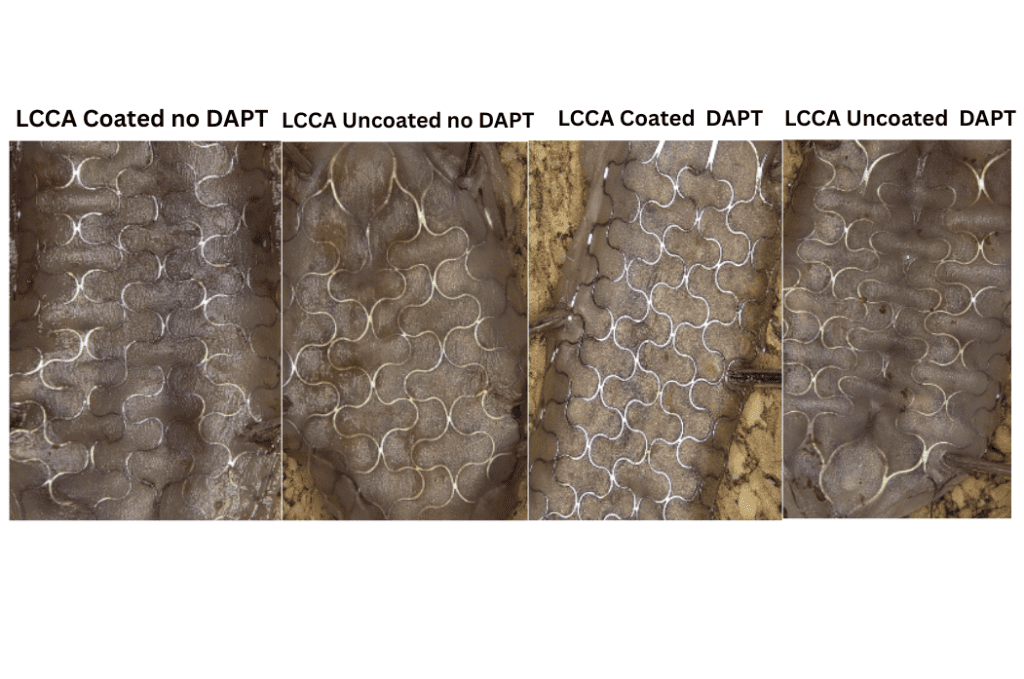
14 days Post Implantation
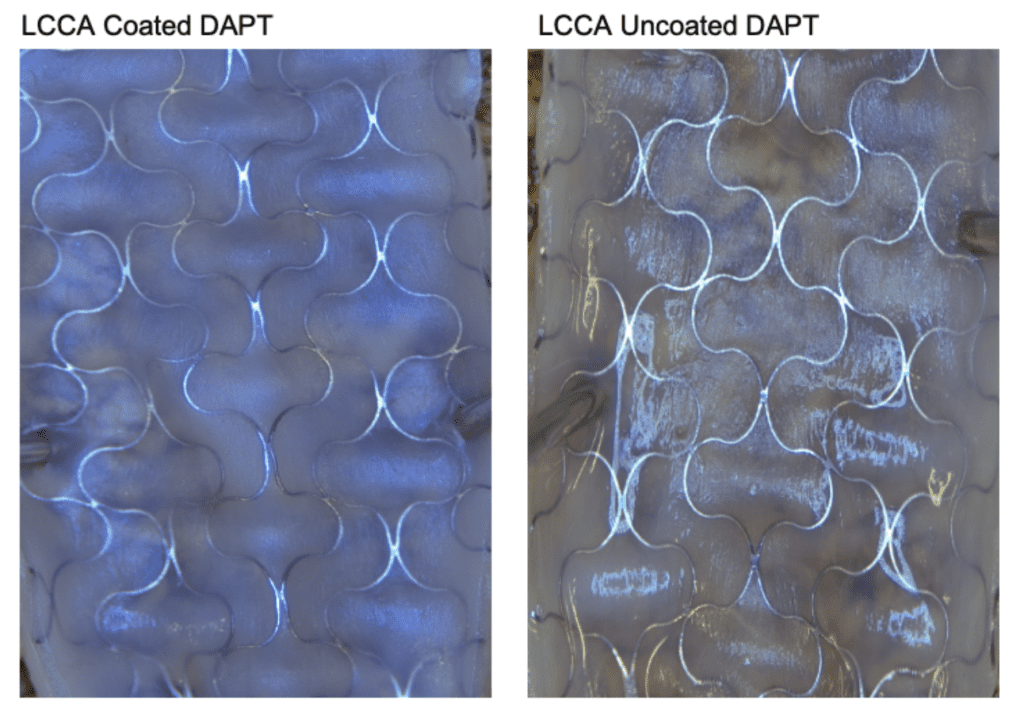
9o days Post Implantation
The Camouflage™ Advantage:
Reduced Thrombosis Risk: By promoting endothelialization, Camouflage™ minimizes the risk of blood clot formation, a common issue with implants like stents and heart valves.
Enhanced Biocompatibility: The rapid formation of a natural endothelial layer reduces immune reactions and inflammation, helping the implant integrate seamlessly with the body.
Improved Long-Term Device Performance: With superior endothelial coverage, devices coated with Camouflage™ experience better long-term stability, reducing the need for revision surgeries and improving patient outcomes.
Faster Healing Time: Camouflage™ accelerates the body’s natural healing process, allowing for quicker recovery and reduced complications post-implantation.
Mechanism of Action
Cell Adhesion: The surface properties of Camouflage™ are engineered to facilitate the adhesion of endothelial cells. This is achieved by allowing a layer of the patients proteins to adhere to the surface and provide a stable surface for cell attachment.
Cell Proliferation: Once endothelial cells adhere to the Camouflage™ coated surface, the coating supports their proliferation and migration. This ensures that the cells can spread across the surface of the implant, forming a continuous and uniform endothelial layer.
Maturation: After the endothelial cells have adhered and proliferated, Camouflage™ supports the maturation of the endothelial layer. This maturation process involves the cells developing into a fully functional barrier that closely resembles the natural endothelium of blood vessels. A mature endothelial layer is essential for maintaining long-term hemocompatibility and reducing the risk of complications.
Factors Influencing Endothelialization
Surface Properties: The surface characteristics of the implant, such as texture and chemistry, can influence endothelial cell adhesion and growth. Camouflage™ works to ensure that the surface area is prepared for cell growth.
Biocompatibility: Camouflage™ makes the material of the implant to be biocompatible to support endothelialization.
Hemodynamics: Camouflage™ is an ultra-thin coating that preserves the hemodynamic design of devices, ensuring optimal blood flow and supporting uninterrupted endothelialization.
By choosing Camouflage™, medical device manufacturers can ensure their products meet the highest standards of biocompatibility and performance, while ultimately improving patient safety and outcomes. Camouflage™ can be applied on Vascular grafts, Cardiovascular implants, Dialysis devices etc.
Next step
Learn how Camouflage™ can transform your medical devices by boosting endothelialization and reducing complications. Contact us for more information or to request a demo of how Camouflage™ works in your specific application.
Why Choose Camouflage™?
With Camouflage™, medical device manufacturers gain access to an advanced coating technology that directly addresses the challenges of endothelialization. Choosing Camouflage™ means choosing:
Safety: Reduced risk of life-threatening complications such as thrombosis and immune reactions.
Performance: Long-term device functionality with fewer complications.
Patient Trust: Devices that integrate seamlessly with the body lead to better patient outcomes and satisfaction.