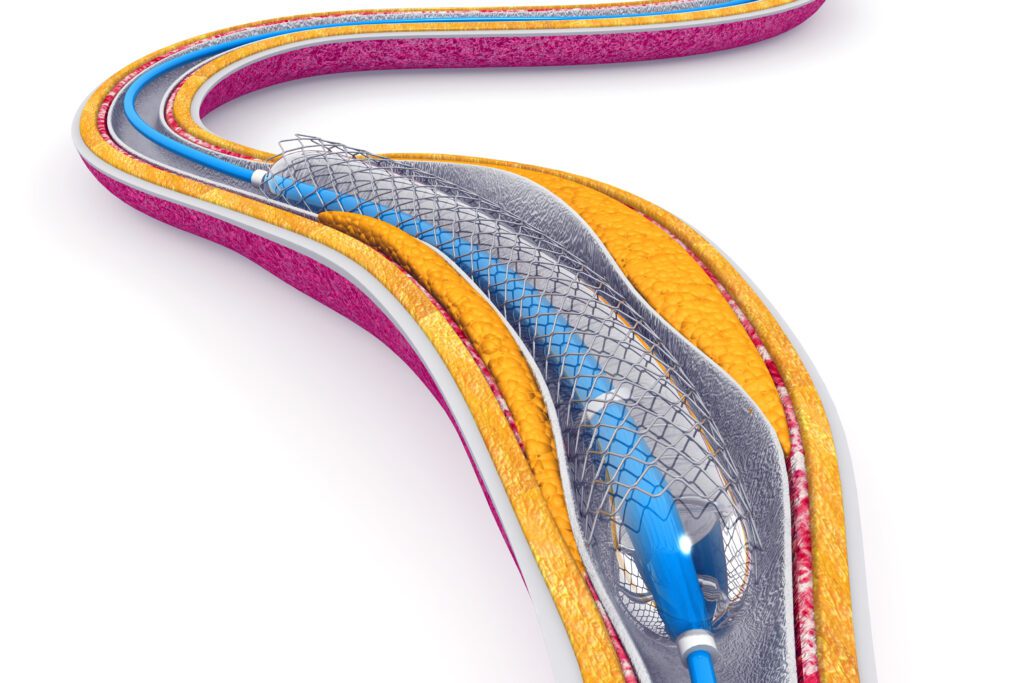
Flow diverters (FDs), a key aspect of Flow diverter development, are endovascular devices used to treat intracranial aneurysms by diverting blood flow away from the aneurysm sac and encouraging vessel healing. While the clinical role of FDs is well established, their design is inherently complex and plays a critical role in determining the safety, performance, and long-term biological outcomes of the device.
This blog outlines the most important elements of FD design – ranging from mechanical construction to biological integration – to support researchers, clinicians, and device developers in understanding the core principles that underpin successful device performance.
1. Wire Material Selection
The choice of base material in FDs is fundamental to their mechanical and biological characteristics. Each material offers different advantages:
- Nitinol – Known for its superelasticity, shape memory, and high flexibility, nitinol is ideal for navigating complex cerebral anatomy.
- Cobalt-chromium – Provides strength and stiffness compared to nitinol making it a suitable choice where structural support is prioritized.
- Platinum – Incorporated to improve radiopacity, platinum enhances visibility under fluoroscopy during deployment and follow-up imaging.
Understanding the principles of Flow diverter development is essential for improving device efficacy and patient outcomes.
In some designs, composite materials, such as drawn filled tubing (DFT) combine a radiopaque core with a flexible outer shell (e.g., platinum core with nitinol shell), allowing optimal visibility and mechanical performance.
2. Braid Geometry and Structural Design
FDs are typically fabricated by braiding 36–96 ultra-fine wires into a mesh tube. The braid geometry significantly affects device behavior:
- Wire diameter – Thicker wires increase radial force, improving vessel wall conformity but reduce flexibility.
- Number of wires – Increasing the number of wires improves flow diversion efficiency and metal coverage but may also increase device stiffness and thrombotic risk.
Braid Angle and PPI (picks-per-inch) – A higher braid angle or PPI results in greater metal surface coverage, promoting aneurysm exclusion; however, it may also reduce flexibility and trackability.
3. Porosity, Metal Coverage, and Pore Density
The porosity of a flow diverter is a critical determinant of how much blood can travel over the device, directly influencing both flow dynamics and biological response:
- Low porosity (50–70%): Promotes intra-aneurysmal blood stasis, facilitating thrombosis and eventual aneurysm exclusion.
- High pore density (13–30 pores/mm²): Enhances flow diversion and encourages endothelialization across the aneurysm neck.
- Metal coverage: Impacts both the mechanical integrity of the device and its ability to support biological integration.
4. Wall Apposition
Wall apposition describes the degree to which the FD conforms uniformly to the inner surface of the vessel wall following deployment. Optimal apposition is critical to both the safety and efficacy of the device. Poor apposition can result in gaps between the device and the vessel wall, creating regions of disturbed blood flow that increases the risk of thrombus formation, endothelial disruption and delayed vessel healing. Optimal wall apposition facilitates neointimal growth across the aneurysm neck and supports long -term vessel remodeling.
Design elements that directly influence wall apposition include:
- Radial Force: Sufficient outward pressure ensures the device maintains consistent contact with the vessel wall.
- Flexibility: Enhanced flexibility allows the device to adapt to the complex geometry of intracranial vessels.
- Oversizing Strategy: Appropriate oversizing ensures adequate wall contact without excessive force, which could otherwise lead to vessel injury.
5. Deliverability
Deliverability refers to the FDs ability to be navigated through complex neurovascular anatomy and deployed accurately at the target site. Several critical design features contribute to optimal deliverability including:
- Microcatheter compatibility – Devices must be compatible with low profile microcatheters (e.g., ≤ 0.027-inch inner diameter) to facilitate access to distal locations while minimizing vessel trauma.
- Low delivery force – Reduced resistance during advancement enhances trackability and minimizes the risk of kinking or vessel damage during navigation.
- Flexibility and Length Recovery – The device must maintain sufficient flexibility to cover highly curved anatomy, while also exhibiting length recovery upon deployment to ensure precise coverage of the aneurysm neck.
6. Radiopacity and Imaging Compatibility
FDs are deployed under fluoroscopic guidance, making radiopacity a critical feature for precise visualization and accurate placement. Key considerations include:
- Radiopaque markers – Commonly made from materials such as platinum and are strategically positioned at the device ends to enable clear visualization during deployment.
- Radiopaque wires or drawn filled tubing (DFT) – enhance continuous device visibility along its entire length, improving navigation through complex anatomy.
7. Surface Modification and Hemocompatibility
The bare metal surfaces of FDs particularly those composed of nitinol can trigger thrombogenic and inflammatory responses upon blood contact. Advanced surface modifications are therefore employed to enhance hemocompatibility and promote vascular healing. Non-drug eluting coatings such as Smart Reactors proprietary Camouflage technology is designed to reduce platelet adhesion and activation, thereby minimizing thrombosis risk while encouraging endothelial cell growth and vessel wall integration. Surface treatments have become particularly important in clinical scenarios where dual antiplatelet therapy may need to be minimized, providing a safer therapeutic option without compromising device performance.
8. Long-Term Integration and Healing
A successful FD design must achieve more than just mechanical deployment; it must actively facilitate biological processes to ensure durable aneurysm treatment and vascular health:
- Effective Flow Diversion – To redirect blood away from the aneurysm sac, reducing haemodynamic stress and promoting thrombus formation within the aneurysm.
- Neointimal Coverage – Across the aneurysm neck, which is critical for permanent aneurysm exclusion and vessel wall healing.
- Prevention of long-term complications – such as in-stent stenosis, thrombus migration or vessel occlusion can undermine patient outcomes.
Conclusion: The Multifaceted Challenge of Flow Diverter Design
Designing an effective FD demands a multidisciplinary approach that seamlessly integrates engineering precision, advanced materials science and a deep understanding of vascular biology. Each design element from the intricacies of braid geometry to the nuances of surface coatings – must be carefully calibrated to achieve an optimal balance between radiographic visibility, flexibility, deliverability and biological healing response.
Smart Reactors Camouflage™ coating enhances hemocompatibility by reducing thrombogenicity and supporting endothelization, all while preserving the devices mechanical properties. This makes it an ideal solution for any modern FD design where surface functionality is just as critical as structural integrity.
Interested in enhancing your flow diverter design with Camouflage™?
Visit www.smartreactors.com or contact our team to explore coating integration options.
Share this post: on LinkedIn