Hemocompatibility is a critical aspect of biocompatibility, particularly for medical devices that come into contact with blood. Blood is a complex biological fluid with essential roles including oxygen transport, immune defense, and tissue repair. Because of this, any surface that interacts with blood must be evaluated for hemocompatibility to ensure it does not trigger harmful responses.
Why Hemocompatibility Testing Is Essential
Medical devices that contact the circulatory system can cause adverse reactions such as clot formation, hemolysis, inflammation, or platelet activation. To assess and limit these risks, hemocompatibility testing is conducted according to ISO 10993-4. This international standard outlines proven in vitro methods for evaluating specific blood-related parameters. Devices are tested using either static or dynamic conditions and compared against control materials and marketed comparators to reduce variability from donor differences.
Static Hemocompatibility Testing
Static testing exposes device materials to blood in stationary conditions. Blood is incubated with the test material and analyzed for specific biomarkers. This approach is a cost-effective initial screening tool and is less complex than dynamic testing. Although it lacks the physiological flow conditions of the circulatory system, static testing can still yield valuable data about surface interactions and compatibility.
Dynamic Hemocompatibility Testing
Dynamic testing mimics the physiological conditions of blood flow and includes the use of rockers, rotators, and the Chandler loop system.
Rockers and Rotators simulate mechanical forces and fluid motion to evaluate how blood interacts with a material. Rockers gently tilt samples back and forth, while rotators apply centrifugal force. These systems provide more realistic insights into blood-material interactions compared to static models.
Chandler Loop Model
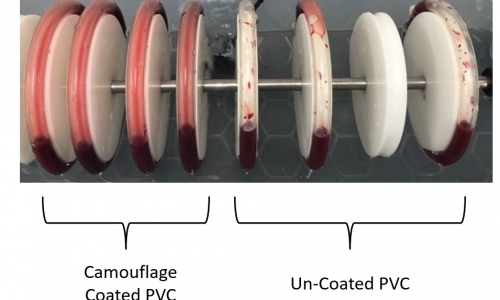
uses a closed-loop system where blood is circulated through tubing containing the device material. The loop is kept at body temperature and continuously circulates blood for a set duration. This method simulates the cyclical and mechanical forces found in the human circulatory system. In tests conducted with Smart Reactors’ Camouflage™ coated tubing, uniform blood adhesion was observed, compared to patchy deposition in uncoated PVC
Biomarkers Evaluated in Hemocompatibility
ISO 10993-4 defines key biomarkers and methods to evaluate device safety and performance in blood-contact situations. These include:
Coagulation is measured by detecting Thrombin-Antithrombin (TAT) complexes using ELISA. TAT formation indicates activation of the clotting cascade, serving as a key marker of thrombogenicity.
Platelet Activation is analyzed through platelet count, shape changes, and biomarkers such as beta-thromboglobulin and Platelet Factor 4. A decline in platelet function or count suggests surface-induced activation.
Hemolysis is evaluated by measuring free hemoglobin released from red blood cells using spectrophotometry. This quantifies the extent of red blood cell membrane damage.
Hematology involves assessing white and red blood cell counts. A reduction in leukocyte or erythrocyte levels may indicate activation or destruction caused by the device surface.
Partial Thromboplastin Time (PTT) assesses intrinsic coagulation pathway activation. Test materials are introduced to platelet-poor plasma, and the time to clot formation is recorded.
Complement System Activation is measured by detecting C3a, C5a, and SC5b-9 complexes through ELISA. These indicate the activation of immune responses and potential inflammation.
Leukocyte Activation is indicated by PMN-elastase-α1-proteinase inhibitor complexes. These markers suggest neutrophil activation and an inflammatory response triggered by the material.hod where the cyanide derivative of hemoglobin is measured through the addition of potassium cyanide and ferricyanide to blood.
Surface Analysis Methods
Microscopy techniques like scanning electron microscopy (SEM) and light microscopy are used to analyze how blood interacts with a material at the surface level. These methods help identify irregularities, roughness, or deposition of platelets and fibrin that may contribute to thrombosis. SEM offers high-resolution imaging for detailed surface characterization.
In Vivo Hemocompatibility Testing
ISO 10993-4 also describes in vivo models for evaluating thrombosis in blood-contacting devices. In the NAVI (non-anticoagulated venous implant) and AVI (anticoagulated venous implant) models, devices are implanted into the veins of animals to assess thrombus formation. Commonly used models include the canine femoral or jugular vein for up to 4 hours.
For long-term studies, heart-lung ECMO models in large animals are used. These simulate veno-arterial extracorporeal circulation over multiple days, allowing evaluation of device performance under clinically relevant conditions.
Benefits of Hemocompatible Coatings
Medical devices made from materials like polypropylene or polymethylpentene may have poor inherent hemocompatibility. Applying hemocompatible coatings helps improve surface performance, reduce thrombosis risk, and promote endothelialization. These coatings support device longevity and patient safety.
Smart Reactors’ Camouflage™ coating is designed to improve hemocompatibility by reducing platelet adhesion and enhancing wettability. Comparative studies in Chandler loop models show that Camouflage™ offers uniform blood interaction, supporting better clinical outcomes.
To learn more, explore our resource page on hemocompatible coatings, where we compare leading technologies and show how Smart Reactors’ surface solutions improve performance for cardiovascular, neurovascular, and dialysis devices.
Share this post: on LinkedIn